THORIUM
Thorium is a soft, very ductile, silver-gray, heavy, metallic element of the actinide series of elements. It is represented by the chemical symbol Th or the isotopic symbol Th232. Thorium metal has a very high melting point, while the oxide, which is also called thoria, has the highest melting point of all the binary oxides. Th232 is the most abundant of the four naturally occurring isotopes of thorium. Th232 emits radioactive alpha particles and has a very long half-life of 1.405 x 1010 years. Daughter products of the Th232 disintegration series produce alpha, beta, and gamma emissions. Most products of the disintegration series have relatively short half-lifes, ranging from 5.75 years to 0.145 seconds. The final decay product of the Th232 series is the stable isotope Pb208. Thorium’s other naturally occuring isotopes,Th228, Th230, and Th234, have half-lifes of 1.9116 years, 75,380 years, and 241 days, respectively.
Thorium was discovered in 1828 by Swedish chemist and mineralogist Jons Jakob Berzelius. He named it thoria, after Thor, the ancient Norse god of thunder. Berzelius isolated the element from a black silicate mineral from the island of Lovo near Brevig, Norway. Subsequently, the black mineral from which thoria was derived was named thorite. Thorium’s radioactivity was discovered independently in 1898 by Madame Marie Curie and C.G. Schmidt.
Thorium is the 39th most abundant of the 78 common elements in the Earth’s crust, at 7.2 parts per million. It is about three times more abundant than uranium and is associated with uranium in igneous rock As the primary thorium minerals are more resistant to geochemical and physical weathering, the thorium/uranium ratio in sedimentary rock is typically higher than its igneous source rock. Thorium occurs in several minerals, the most common being monazite and thorite.
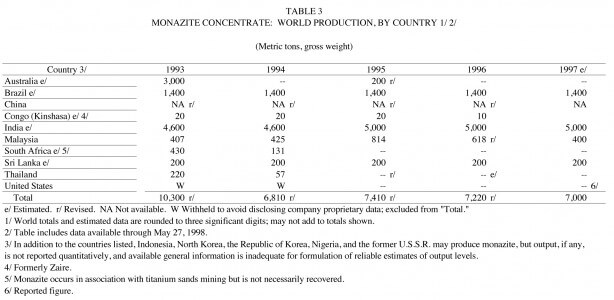
Domestic consumption of refined thorium products increased in 1997, according to the U.S. Geological Survey (USGS). The value of thorium metal and compounds used by the domestic industry was estimated to be about $300,000. Thorium production was primarily from the rare-earth-thorium-phosphate mineral, monazite, a byproduct of processing heavy-mineral sands for titanium and zirconium minerals or tin minerals. Thorium compounds were produced from monazite during processing for the rare earths. Only a small portion of the thorium produced was consumed; most was discarded as waste. The major monazite-producing countries were Brazil, China, India, Malaysia, and Sri Lanka. Essentially all of the thorium compounds, metal, and alloys used by the domestic industry were derived from imports, company stocks, or material sold from U.S. Government stocks.
Limited demand for thorium, relative to the rare earths, continued to create a worldwide oversupply of thorium compounds and residues. Most major rare-earth processors have switched feed materials to thorium-free intermediate compounds. Excess thorium, not designated for commercial use, was either disposed of as a radioactive waste or stored for potential use as a nuclear fuel or other application. Principal nonenergy uses have shifted from refractory applications to welding electrodes and lighting.
Problems associated with thorium’s natural radioactivity represented a significant cost to those companies involved in its mining, processing, manufacture, and use. Increased costs to comply with environmental regulations, potential legal liabilities, and the costs to purchase storage and waste disposal space were the principal deterrents to its commercial use. According to industry sources, health concerns associated with thorium’s natural radioactivity have not been a significant factor in switching to alternative nonradioactive materials.
Legislation and Government Programs
The calendar year 1997 included parts of the U.S. Government fiscal years (October 1 to September 30) 1997 and 1998. Public Law 104-201, the National Defense Authorization Act for Fiscal Year 1997, was enacted on September, 23, 1997. It did not change the previous authorization for the disposal of all stocks of thorium nitrate in excess of the National Defense Stockpile (NDS) goal of 272,155 kilograms (600,000 pounds). The National Defense Authorization Act for Fiscal Year 1998, Public Law 105-85, also known as the “Strategic and Critical Stock Piling Act,” was enacted on November 18,1997. The revised annual material plan proposed the disposal of up to 453,592 kilograms (1,000,000 pounds) of thorium nitrate in fiscal year 1998. It did not change previous authorizations for the disposal of 2,946,185 kilograms (6,495,225 pounds) of thorium nitrate classified as excess to goal.
Production
Domestic mine production data for thorium-bearing monazite were developed by the USGS from a voluntary survey of U. S. operations entitled “Thorium.” The one mine to which a survey form was sent responded. Thorium was not produced in the United States in 1997, however, the mine that had previously produced thorium-bearing monazite continued to operate and maintained capacity on standby. Monazite was last produced in the United States in 1994.
Essentially all thorium alloys and compounds used by the domestic industry were derived from imports, company stocks, or materials sold from U.S. Government stockpiles. Domestic companies processed or fabricated various forms of thorium for nonenergy uses such as ceramics, magnesium-thorium alloys, refractories, and welding electrodes.
Consumption
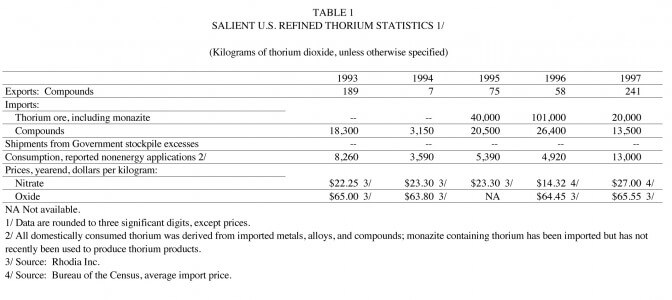
Statistics on domestic thorium consumption are developed by surveying various processors and manufacturers, evaluating import-export data, and analyzing Government stockpile shipments. (See table 1.)
Domestic thorium producers reported consumption of 13.0 metric tons of thorium oxide equivalent in 1997, an increase from the 1996 level of 4.92 tons. The increase was primarily the result of increased demand for thorium in a catalyst application. Nonenergy uses accounted for essentially all of the total consumption.
Thorium oxide (thoria) has the highest melting point of all the binary metal oxides at3,300°C. This property contributed to its use in several refractory applications. High-temperature uses were in ceramic parts, investment molds, and crucibles.
Thorium nitrate was used in the manufacture of mantles for incandescent “camping” lanterns, including natural gas lamps and oil lamps. Thorium mantles provide an intense white light that is adjusted towards the yellow region by a small addition of cerium. Thoriated mantles were not produced domestically due to the development of a suitable thorium-free substitute.
Thorium nitrate also was used to produce thoriated tungsten welding electrodes. Thoriated tungsten welding electrodes were used to join stainless steels, nickel alloys, and other alloys requiring a continuous and stable arc to achieve precision welds.
The nitrate form was also used to produce thoriated tungsten elements used in the negative poles of magnetron tubes. Thorium was used because of its ability to emit electrons at relatively low temperatures when heated in a vacuum. Magnetron tubes were used to emit electrons at microwave frequencies to heat food in microwave ovens and in radar systems used to track aircraft and weather conditions.
Thorium was used in other applications as catalysts, electron emitting-tubes, elements in special use light bulbs, fuel cell elements, high-refractivity glass, photo conductive films, radiation detectors, and target materials for X-ray tubes.
In metallurgical applications, thorium was alloyed primarily with magnesium. Thorium metal has a high melting temperature of 1,750°C and a boiling point of about 4,790°C. Magnesium-thorium alloys used by the aerospace industry are lightweight and have high strength and excellent creep resistance at elevated temperatures. Thorium-free magnesium alloys with similar properties have been developed and are expected to replace most of the thorium-magnesium alloys presently used. Small quantities of thorium were used in dispersion-hardened alloys for high-strength, high-temperature applications.
Thorium was used as a nuclear fuel in the thorium-232/ uranium-233 fuel cycle. No foreign or domestic commercial reactors are operating with this fuel cycle. The use of thorium as a nuclear fuel is not expected to grow due to the current availability of low-cost uranium.
Stocks
Government stocks of thorium nitrate in the NDS were 3,216,828 kilograms (7,091,891 pounds) on December 31, 1997. The NDS shipped 1,724 kilograms (3,800 pounds) for waste disposal research in 1997. The NDS inventory included 273,181 kilograms (602,262 pounds) of thorium nitrate allocated to meet the NDS goal requirements and 2,943,646 kilograms (6,489,629 pounds) classified as excess to the goal. No stocks of thorium nitrate were sold during the year.
The U.S. Department of Energy’s (DOE) inventory at yearend was 535,000 kilograms of thorium oxide equivalent contained in metal and various compounds. The DOE contractor, Fernald Environmental Restoration Management Company (FERMCO), a subsidiary of Huor Daniel’s Fernald, shipped 140,000 kilograms of thorium oxide in 1997 FERMCO also shipped to waste disposal an estimated 4,200 kilograms of thorium oxide equivalent contained in ore.
Prices
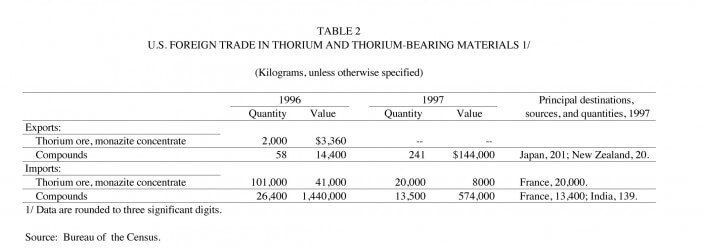
The price of monazite concentrate, typically sold with a minimum 55% rare-earth oxide including thoria, free-on-board (f.o.b.) as quoted in U.S. dollars and based on U.S. import data, was $400 per ton. The 1997 monazite price increased from the previous years price range, as converted from Australian dollars (A$), of US$244-US$285 (Metal Bulletin, 1997). The 1997 price of monazite on a contained rare-earth oxide basis sold for $0.73 per kilogram.
Thorium oxide prices quoted by Rhone-Poulenc Basic Chemicals Co. (Rhodia as of January 1, 1998) decreased slightly or were unchanged in 1997. At yearend 1997, thorium oxide prices per kilogram f.o.b. Shelton, CT, were $82.50 for 99.9% purity and $107.25 per kilogram for 99.99% purity.
Thorium alloy prices, previously quoted by Reade Manufacturing Co., a division of Magnesium Elektron, Lakehurst, NJ, were no longer available because of depletion of the company’s stocks.
World Review
Thorium demand continued to remain depressed as industrial consumers expressed concerns with the potential liabilities, the costs of complying with environmental monitoring and regulations, and cost increases at approved waste disposal sites.
Outlook
Nonenergy uses for thorium in the United States have decreased substantially over the past 7 years. Domestic demand is forecast to remain at current depressed levels unless low-cost technology is developed to dispose of residues. Manufacturers have successfully developed acceptable substitutes for thorium-containing incandescent lamp mantles, paint and coating evaporation materials, magnesium alloys, ceramics, and investment molds. The traditionally small markets for thorium compounds, welding electrodes, and lighting, are expected to remain the leading consumers of thorium compounds through the end of the millennium. Thorium’s potential for growth in nonenergy applications is limited by its natural radioactivity. Its greatest potential exists in energy applications as a nuclear fuel or subatomic fuel in an industry that accepts radioactivity. In the long term, high disposal costs, increasingly stringent regulations, and public concerns related to thorium’s natural radioactivity are expected to continue to depress its future use.
Reference Cited
Metal Bulletin, 1996, Non-ferrous ores: Metal Bulletin [London] no,. 8141, December 31, p.25.
SOURCES OF INFORMATION
U.S. Geological Survey Publications
horium. Annual Mineral Industry Surveys.
Thorium. Ch. In Mineral Commodity Summaries, annual.
Thorium. Ch. In Minerals Yearbook.
Nuclear Fuels. Ch. In United States minerla resources, U.S.
Geological Survey Professional Paper 820, 1973.
Other
Uranium Industry Annual 1997, U.S. Department of Energy.
Thorium. Ch. In Minerla Facts and Problems, U.S. Bureau of
Mines Bulletin 675, 1985.