In 1994, R. Sundaresan and J.Bockris (Texas A&M) reported that they had observed “Anomalous Reactions During Arcing Between Carbon Rods In Water:
“Spectroscopically pure carbon rods were subjected to a carbon arc in highly purified water. The arc current varied from 20 to 25 A and was passed intermittently for several hours. The original carbon contained ~ 2 ppm Fe. The C rods remained cool to the touch at >2 cm from their tips. Absorption of iron from water or the surrounding atmosphere was established as not being the cause of the increase of iron. There is a weak correlation between the iron formed and the time of passage of current.
“When dissolved O2 was replaced by N2 in the solution, no iron was formed. Hence, the mechanism
26C12 + 2 8O18=26Fe56 + 2He4
was suggested as the origin of the iron. The increase in temperature of the solution was consistent with expectation based on this reaction.”
The Fe which is produced by this transmutation is stainless. It does not rust easily. It has also much less reaction to heat than ordinary iron, due to its composition of 2 Si (silicon) atoms. This iron was name G.O.S. (George Ohsawa Steel), given the initials of George Ohsawa by the scientists who worked with this transmutation. All results of the transmutation of Fe have been carefully examined and analyzed by several methods including: magnetic inspection, spectroscopic analysis, chemical analysis, and examination by reagent, confirmed by authoritative testing agencies.
Also in 1994, another group of researchers (M. Singh, et al.) at the Bhabha Atomic Research Centre (Bombay) reported their “Verification of the G. Ohsawa Experiment for Anomalous Production of Iron from Carbon Arc in Water:
“A direct current arc was run between ultrapure graphite electrodes dipped in ultrapure water for 1-20 hours. The graphite residue collected at the bottom of the water trough was analyzed for Fe content by a conventional spectrographic method… The Fe content was fairly high, depending on the duration of the arcing… The results showed large variations in Fe content (50 to 2000 ppm) in the C residue. In the second series of experiments… with the water trough fully covered, the amount of Fe in the carbon residue decreased significantly (20-100 ppm). Here also there were large variations in the iron concentration in the residue, although the experiments were performed under identical conditions. Whether Fe is really being synthesized through transmutation from C and O as suggested by George Ohsawa or is getting concentrated to different degrees through some other phenomenon is not currently clear. The Fe in the C residue was also analyzed by mass spectroscopy for the abundance of various isotopes… Besides Fe, the presence of other elements like Si, Ni, Al, and Cr was also determined in the C residue, and it was found that the variation of their concentrations followed the same pattern as that of Fe.”
Anomalies of Generated Molecules
Santilli’s main hypothesis for the resulting gases anomalies is that, at the time of their formation under an electric arc, gases H2, CO, CO2, O2, etc. do not have a conventional structure because the orbits of their valence electrons, and maybe also their necleus shells are mostly polarized in a plane due to the very intense magnetic field surrounding the electric arc (of the order of 10 Tesla or more). In turn, such a polarization implies the creation of strong magnetic moments, resulting in new magnetic bonds constituting magnecules.
The experimental verification of the fuel gas requires the detection of a number of anomalies that can be summarized as follows. All these anomalies have been experimentally verified.
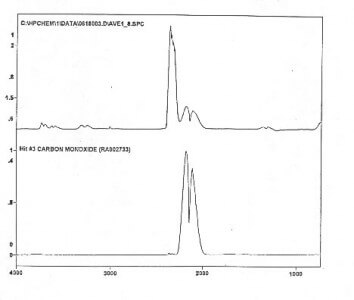
Anomaly 1: Appearance of unexpected heavy MS peaks.
Fuel gas molecules, referred as magnecules are generally heavier than the heaviest molecule in a given gas. Peaks in the GC-MS are therefore expected in macroscopic percentages with molecular weights bigger than the heaviest molecule. These heavy composites should not provide MS peaks according to quantum chemistry, thus constituting an anomaly. As an example, by ignoring heavy compounds in parts per million [ppm], MagnegasesTM should have no large peak in the GC-MS with more than the CO2 molecular weight of 44 a.m.u. The existence of heavier large peaks would establish this first anomaly.
Anomaly 2: “Unknown” character of the unexpected heavy peaks.
To provide the initial premises for magnecules, the peaks of Anomaly 1 should result in “unknown” in the search by the GC-MS computer in its memory banks of conventional molecules, usually including about 150,000 molecules.
Anomaly 3: Lack of IR signature of the “unknown” peaks.
Another necessary condition to have magnecules is that the “unknown” peaks of Anomaly 1 should have no infrared signature at all. According to established evidence, all gases with a valence bond must have a well defined infrared signature [with a few exceptions of spherically symmetric molecules, such as hydrogen]. In the event the peaks of Anomaly 1 do have an infrared signature, they can be constituted by new yet conventional molecules not identified before. The only infrared signatures of any given gas magnecule should be those of the conventional molecules and atoms constituting the cluster itself. As an illustration, the only admissible infrared signatures of magnecule {O2}x{O2} are those of the conventional molecules O-O and C-O.
Anomaly 4: Mutation of conventional IR signatures.
The infrared signatures of the molecules constituting a magnecules are expected to be mutated, in the sense that the shape of their peaks is not the established one. This is another anomaly of magnecules expected from the polarization of the orbits of the valence and other electrons. In fact, this polarization implies space distributions of the orbitals different than the conventional ones, thus resulting in a deformation of the shape of the IR peaks. Moreover, the same polarizations are expected to create additional strong bonds within a conventional molecule, that are expected to appear as new IR peaks. Still in turn, such an internal mutations of conventional molecules have far reaching scientific and technological implications, as will be shown.
Anomaly 5: Mutation of magnecules.
While molecules preserve their structure at conventional temperatures and pressures, this is not the case for magnecules, that are expected to mutate in time, that is, to change the shape of the MS peaks due to change in their constituents. Since we are referring to gases whose constituents notoriously collide, magnecules can break-down into parts during collisions, which parts can then recombine with other magnecules to form new clusters. Alternatively, magnecules are expected to experience accretion [or emission] of polarized conventional atoms or molecules without necessarily breaking down into parts. It follows that the peaks of Anomaly 1 are not expected to remain the same over a sufficient period of time for the same gas under the same conditions.
Anomaly 6: Mutated physical characteristics.
Magnetically polarized gases are expected to have mutated physical characteristics because the very notion of polarization of the orbits implies a smaller average molecular volume. Mutations of other physical characteristics are then consequential.
Anomaly 7: Anomalous adhesion.
Magnetically polarized gases are expected to have anomalous adhesion to walls of disparate nature as compared to the same unpolarized gas. This is due to the well known property that magnetism can be propagated by induction, according to which a magnetically polarized molecule with a sufficiently intense magnetic moment can induce a corresponding polarization of valence [and_or other] electrons in the atoms or molecules constituting the walls surface. Once such a polarization is created by induction, magnecules can have rather strong magnetic bonds to said walls.
Anomaly 8: Increased penetration through substances.
Magnetically polarized gases are expected to have anomalous absorption or penetration through other substances. This is first due to the reduction of the average molecular volume with inherent increase of permeability, as compared to the same unpolarized gas. The second reason is the magnetic induction of the preceding anomaly.
Anomaly 9: Increased energy release.
Magnetically polarized gases are expected to have thermochemical reactions with macroscopic increases of energy releases, as compared to the same reactions among unpolarized gases, an expected anomaly that, alone, has large scientific and industrial significance.