Chapter I – The Problems of Heat
1. Old and New Ideas on the Causes of Heat
Hardly any scientific subject has called forth so many researches as heat. Thanks to them, thermodynamics and the energetic theory, which are derived from it, have become precise and fertile sciences.
But if we simply ask ourselves what these researches have revealed as to the causes of heat, we are bound to acknowledge that we are hardly more advanced than we were a century ago. We cannot find much to add to the following lines written by the illustrious Humpry Davy, a hundred years ago:
“Since all matter may be made to fill a smaller volume by cooling, it is evident that the particles of matter must have space between them; and since every body can communicate the power of expansion to a body of a lower temperature, that is, can give an expansive motion to its particles, it is a probable inference that its own particles are possessed of motion; but as there is no change in the position of its parts as long as its temperature is uniform, the motion, if its exists, must be a vibratory or undulating motion, or a motion of the particles round their axes, or a motion of particles round each other.
“It seems possible to account for all the phenomena of heat, if it be supposed that in solids the particles are in a constant state of vibratory motion, the particles of the hottest bodies moving with the greatest velocity, and through the greatest space; that in fluids and elastic fluids, besides the vibratory motion, which must be conceived greatest in the last, the particles have a motion round their won axes, with different velocities, the particles of elastic fluids moving with the greatest quickness; and that in ethereal substances the particles move round their own axes, and separate from each other, penetrating in right lines through space. Temperature may be conceived to depend upon the velocities of the vibrations; increase of capacity on the motion being performed in greater space; and the diminution of temperature during the conversion of solids into fluids or gases, may be explained on the idea of the revolution of particles round their axes, at the moment when the body becomes fluid or aeriform, or from the loss of rapidity of vibration, in consequence of the motion of the particles through greater space”.
At the present day, as in the time of Davy, we suppose that heat must be the consequence of the movements, vibratory, rotary, etc., of the particles of matter. All researched on the structure of atoms have justified the existence of the movements. Every atom is now compared to a solar system. Naturally we never observe these movements, and mechanical considerations alone lead us to suppose them identical with those of the planets around the sun.
Every component particle of these atoms must be animated by two movements: (1) rotation of the particle on itself; (2) Revolution round a center. These movements may vary, as may the speed of translation, and also the diameter of the orbit traversed. By compelling the molecules to move nearer to or farther from each other, they explain the dilation of bodies by heat and their contraction by cold.
The variations of equilibrium by these elements in motion should produce magnetism, electricity, and heat, but we are entirely ignorant of the way these forces are generated.
We are able to measure heat without knowing anything of its essence. The expression “a quantity of heat” constitutes an arbitrary notion representing the measurement of an effect the cause of which is unknown. “It is nothing else”, writes M. Duhem, “than the measurement given by the calorimeter, and is not otherwise defined. The quantity of heat which is disengaged in a modification is, by definition, a quantity proportional to the weight of water which this modification would raise from the temperature of zero to that of one degree”. The insufficiency of such a conception is evident.
Physicists have at length, however, laid aside this problem of the causes of heat, and, without inquiring how movements can be transformed into heat, they have confined themselves to endeavoring to determine their nature. Although the problem has been taken in hand by such physicists as Clausius and Helmholtz, no success has crowned their efforts. Assimilating, to simplify matters, the elements of bodies to material points in motion, they have admitted that the average force of this movement was proportional to the temperature, and have endeavored to deduce from it the laws of thermodynamics, and, notably, the principles of Carnot, by means of the theorems of mechanics. It is pretty much generally admitted nowadays that this attempt has completely failed. It has furnished, moreover, no sort of hint either as to the variations of trajectory described by the particles of bodies, according to their solid, liquid, or gaseous state, not on the results of their reciprocal actions.
The early physicists regarded the problem in a much simpler way. For them it was a fluid impregnating all bodies and disengaging itself by combustion. This theory, called the phlogistic, was much shaken when Lavoisier proved that, far from losing weight by combustion, bodies on the contrary gained it. Yet at the time of Carnot, heat was still considered to be a fluid that bodies could part with or absorb, and only differing from the old phlogiston by its imponderability.
In the long run physicists gave up the idea of a caloric fluid; but after taking infinite trouble, for more than 50 years, to substitute the mechanical theory of heat produced by movement for that of the conception of a fluid, they seem now — in a rather roundabout way — about to return to the latter. As Prof de Heen has very justly remarked, “The old idea of mixing the phlogistic fluid with matter is identical with the one currently accepted, which consists in mixing with matter electric corpuscles”. There is as much reason for imagining atoms of heat as there is for imagining atoms of electricity.
The ancient idea that heat was a kind of fluid has been very fertile. Without it, Sadi Carnot would, perhaps, never have thought of comparing the flow of heat to that of a liquid, and, no doubt, would never have discovered the principle which bears his name and has so deeply modified the direction of the sciences of physics and chemistry.
It must be fully recognized, moreover, that if physicists and chemists reject the idea of assimilating heat to a fluid, they nearly always treat it as if it really were one. Chemists constantly speak of heat as absorbed or liberated by a body. According to them, when a combination is formed it should keep this heat indefinitely until it is destroyed. It then gives it up in quantity exactly equal to that absorbed. Physicists, for their part, tell us that when a body is heated it absorbed heat and restores it as it cools. We should express ourselves no otherwise if heat were really a fluid.
The mathematicians themselves often employ similar language. All their formulas have been framed as if heat were constituted by a fluid. Laplace, Poisson, Lame, etc., assimilated caloric to an expansive fluid, and the temperature of the particles to the tension of the fluid in them. The variations of heat were explained by changes between these particles of caloric proportional to the difference of their respective temperatures. At the present day, when heat is considered to be a vibratory movement of the particles of matter, we often still continue to argue as if it were a fluid, of which it possesses, in fact, many of the properties.
“The analogy existing between the propagation of heat in athermanous bodies and the filtration of fluids through porous masses is so close”, writes M. Bousinesq in his Theorie Analytique de la Chaleur, “that we might seek to obtain from it a mechanical theory of conductivity if there were such a thing as a caloric fluid”.
We are not certain, moreover, that this fluid does not exist. Electrons are beginning to be made to play a great part in calorific phenomena. After having brought us back to the old electric fluid, they are perhaps going to revive the caloric fluid. For the moment our ignorance on this point is complete.
2. Changes of State of Bodies Under the Influence of Heat and Variations of Energy Resulting Therefrom
The effects of heat on matter are of daily observation. The simple examination of the movements of the thermometer column shows that bodies dilate with heat and contract with cold. The sensitiveness of matter is such, that a variation in temperature of the millionth of a degree suffices to modify its electric resistance in a fashion appreciable by experiment. The slightest oscillation in the ether causes it to vibrate and radiate. There is thus a continuous exchange of energy between matter and the ether.
It is no longer possible at the present day to consider matter independently of its surroundings. The variations of the latter regulate its equilibria and also its form, rendering it solid, liquid, or gaseous. Matter corresponds to a state of equilibrium between its internal energies and the external ones which surround it.
The movements of rotation and of revolution of the elements of the atoms unceasingly vary under the action of heat. It modifies not only their speed of rotation, but also the diameters of the orbits traversed. When these increase, the particles of bodies move farther and farther apart, the molecular attractions which constitute cohesion are overcome, and matter passes first into the liquid, and then into the gaseous state. During these changes, bodies absorb determinate proportions of heat which they restore in absolutely equal quantity when they return to their primary state. The energy absorbed by matter being then exactly given back, we should be justified in believing that matter has never either created or destroyed it.
Heat may, from the physical point of view, be defined as a mode of energy producing the change of volume of bodies, and therefore their dilation. This dilation represents an excellent means of measuring it, but the thermometer, which is based on this property, can only indicate a small part of the heat supplied to a body. When, for example, we heat matter to make it change its state — that is, to liquefy it — we produce three effects, of which only one is revealed by the thermometer: (1) we increase its temperature; (2) we change the internal disposition of its molecules — that is to say, we affect an internal work which is not revealed by the thermometer; (3) we change its volume — that is to say, we effect an external work against external pressure, which, again, is not revealed by the thermometer. It is therefore only a part of the heat produced which has served to change the temperature of the body.
We can, on the other hand, cause the temperature of a body to vary without supplying or abstracting heat from it. This is observed in the operations called adiabatic, for instance, when a gas is compressed in a receptacle impermeable to heat. The temperature is increased by the transformation into heat of the work affected.
The caloric energy necessary to compel bodies to change their state is considerable. To transform ice at 0° C into water at the same temperature, as much heat must be given it as would raise by one degree 80 times its weight of water, or 80 calories. If the water again freezes, it restores the heat absorbed. To transform water at 100° C into steam of the same temperature, the necessary work is more considerable still, since this transformation requires 537 times as much heat as would raise the same quantity of water one degree. As before, these 537 calories are easily restored when the molecules draw together to pass again into the liquid state — that is to say, when the steam at 100° C condenses into water likewise at 100° C.
To show the magnitude of the energies thus displaced, Tyndall gives the following examples: — The heat resulting from the combination of 1 kg of hydrogen with 8 kg of oxygen would raise by 1° C the temperature of 34,000 kg of water which corresponds to more than 14 million kilogram-meters. The condensation in water of the 9 kg of steam formed by this combination, represents a work of more than 2 million kilogram-meters. If, by continuing to run down this scale we bring the water to the solid state by lowering its temperature, it would still produce more than 700,000 kilogram-meters.
The figures representing the forces necessary to modify the molecular states are evidently considerable when judged by our usual units of energy, but they are immensely feeble compared with the intra-atomic forces of which we have elsewhere studies the magnitude.
We must bear in mind from what precedes the constancy of the figures representing the caloric energy displaced in the different variations of the state of matter. That which it absorbs in order to pass from one state to another is always rigorously given back when it returns to its first state. There are, then, simple displacements of energy without destruction or creation.
This fact, so constantly observed, seemed a very solid argument in favor not only of the conservation of energy, but also of the important notion that matter and energy are two very distinct things, the first coming as a support to the second, but never creating it.
My readers know that these principles have been overthrown. Practically, however, the ancient notions retain all their values. For, if matter is an enormous reservoir of energy, and is able to disappear by transforming itself into energy, we do not yet know how to extract from it any but insignificant quantities of this last.
3. Can Heat Serve as the Measure of All Forms of Energy?
In all the changes of state of bodies, we have spoken solely of the heat absorbed or liberated, without troubling ourselves about the other forms of energy. Formerly these were ignored, but the deeper study of the laws of electrolysis having shown that the majority of chemical changes are accompanied by the production of a rigidly constant quantity of electricity for each reaction, it follows that these reactions can be expressed in units of electricity quite as well as in units of heat. The tendency of the present day is to measure reactions by the quantity of electricity displaced rather than by the quantity of heat brought into play. The generation of heat and electricity proceeds by nearly parallel steps, so that we may ask ourselves whether these forces may not be secondary manifestations of unknown energies of which we only perceive the transformations. Chemical energy, for example, is perhaps as different from the electricity and the heat it generates as these last are from friction which can also generate them.
Heat being very early known, and all forces appearing able to transform themselves into heat, it was natural to take it as the unit of measurement. When radiations are allowed to fall on an absorbent surface, we consider those equivalent which produce the same amount of heating. In this way the division of energy in the luminous spectrum has been studied. But it now appears that some very active energies can be manifested under other conditions than heat, and cannot, in consequence, be measured by it. The temperature becomes less and less as we advance towards the extreme ultraviolet in the solar spectrum, and ends by being so minute that it is only perceptible to instruments of excessive sensitiveness. If we confined ourselves to caloric measurements it might be said that energy is almost nil at this end of the spectrum. Now, it is, on the contrary, extraordinarily active, for it dissociates the most resisting bodies, and transforms them into a torrent of particles of the family of the cathode rays.
There are therefore forms of energy which cannot be reduced to heat, and which in consequence heat cannot help us to measure. This very important point will certainly someday attract the attention of physicists.
4. The Conception of the Absolute Zero
The movements of the particles of heated bodies are communicated to substances in contact with them, and cause their volume to change. It is on this fact that the thermometer is based. Plunged into a more or less heated medium, it indicates the difference of temperature between this medium and that of the melting of ice taken as zero during the graduation of the instrument.
This zero is evidently a very arbitrary one, since we might have taken as the starting point of the graduation the point of fusion of any body whatever. All our zeros — such as, for instance, that of electric tension — are equally conventional starting points.
Physicists, however, have for a long time been led to conceive for heat a zero which does indeed deserve the name of absolute which is given to it, since the bodies brought to this temperature would no longer retain any caloric energy. This conception was formed at the time when heat was considered a fluid. The temperature at which bodies would expel all their provision of this fluid constituted the absolute zero.
The theoretic discussions enabling it to be fixed have been numerous. Laplace and Lavoisier placed the absolute zero between 1500 C and 3000 C below melting ice. Dalton fixed it at 1500° C. The reasons for these different conclusions were, however, very unconvincing.
Although it has been abandoned, the theory of the materiality of heat has continued to weigh on the minds of physicist. Considerations drawn from the study of thermodynamics have led Lord Kelvin to adopt for the absolute zero the figure of -273° C, already deduced from the consideration that, as gases contract by 1/273 of their volume per degree, at 273° below the ordinary zero they could contract no further.
According to the conception of the absolute zero, bodies would at -173° C contain no more heat. If heat be only the consequence of the movements of the particles of matter, as is generally admitted, these movements would cease at absolute zero. With this cessation would also disappear, no doubt, the other forces, such as cohesion. One does not, then, very well see what would become of matter. Several physicists, however, at the present day consider the absolute zero as a theoretical and unattainable limit which is merely a datum for calculations.
This theory is much earlier than the date of the discovery of the existence of intra-atomic energy. We may suppose, in strictness, that relatively weak intra-molecular energies may disappear at a certain temperature, but it is impossible to imagine the vanishing of intra-atomic energies. They are so considerable, in fact, that to annul them would require force immeasurably superior to any we have at our disposal. If by any means whatever, such as lowering of the temperature, we succeeded in profoundly disturbing the internal equilibria of the elements always in vibration and rotation, of the atoms of a fragment of matter, they would be disaggregated and would return to the ether.
In this chapter, devoted to the study of heat, we have not had to trouble ourselves with the sensation designated by this term. “That which to our sensations is heat”, says Locke, “is objectively only movement”. The physicists study these movements, but without having yet succeeded in explaining them. Heat is a chapter of physics of which a few fragments are precise, but which is chiefly composed of uncertainties. We shall see the number of these increase when studying the relations of the movements of matter produced by heat with those ethereal ones which these movements generate.
Chapter II – Transformation of Movements of Matter in Vibrations of the Ether — Radiant Heat
1. Nature of Radiant Heat — Absorption and Transformation by Matter of the Vibrations of the Ether
The classic term of radiant heat is one of the most erroneous in physics, notwithstanding its apparent accuracy. If we draw near to a fire, it warms us; it therefore radiates something. What can this something be, if not heat?
It took a very long time to discover that a heated body does not radiate anything resembling heat. It is now known that it produces vibration of the ether, which, in themselves, have no temperature, and that it warms us at a distance because the vibrations of the ether generated it by being affected by the molecules of the air or the bodies before it, generate heat. These vibrations are not heat, but simply a cause of heat, as is any movement whatever.
This confusion of radiant heat with the heat of bodies, which the textbooks stillperpetuate, for a long time prevented us from recognizing the identity of radiant heat and light, formerly considered to be two different things.
That which we call by the very improper name of radiant heat has for its sole origin the vibrations of the ether. These can produce heat when their movement is destroyed, as does a stone by its impact, but they do not possess, I repeat, any temperature of their own. This is easily proved by interposing a lens of ice in the path of a pencil of radiant heat. However intense the pencil may be, the lens is not melted, while a piece of metal placed in its focus will become incandescent. The ether having no temperature, and the ice being very transparent to its vibrations, they have passed through the ice without melting it. As the metal, on the other hand, stops these vibrations, it becomes incandescent by absorbing and immediately restoring them under the form of other vibrations, becoming in its turn a source of that radiant heat without temperature, the effects of which have just been pointed out.
Since the vibrations o the ether, called by the name of radiant heat, can only produce heat after their absorption by a body, it is evident that in the celestial spaces, where an atmosphere like that surrounding the earth does not exist, an absolute cold must reign in the neighborhood of incandescent stars, such as the sun. The thermometer dipped into these spaces would, however, mark there a very high temperature, because it would intercept the vibrations of the ether. The temperature recorded by it would not be that of the ambient medium, but its own temperature. Ice would not melt, because it allows the vibrations of the ether to pass without stopping them. Metal would become incandescent, because it absorbs the same vibrations.
Life is only possible on our globe by reason of the absorption of the vibrations of the ether by the atmosphere and the earth; if these last were transparent to them, a very intense cold would reign on the surface of our planet.
All the chemical reactions which take place in the interior of vegetables, notably the transformation of carbonic acid into carbon, have their origin in this absorption.
The vibrations of the ether when absorbed by a body may be retained by it, and become the origin of various chemical transformations. They are thus fixed until the time when, by decomposing the body — that is to say, by bringing it back to its former state — we make them reappear under the form of heat. We have here one proof the more of the intimate relations of the ether with the matter, and of the exchanges of energy of which it is the seat.
If the vibrations of the ether absorbed by matter are not used in chemical transformations, they only raise the temperature of bodies, and disappear by radiation with a rapidity dependent on the structure of these bodies or of the substances with which they are covered. A vessel of polished metal loses its heat by slow degrees, and this is why we employ it to keep liquids at a high temperature. The same metal, if covered with lacquer, on the other hand, rapidly parts with its heat. These are facts long known, which Lister in other days put in evidence by his cube full of boiling water, the faces of which were composed of different metals. Each face radiated different quantities of heat.
All these facts find a rudimentary explanation in the phenomenon of acoustic resonance. A tuning fork insensible to the most violent noise will vibrate if struck by sound waves of suitable periods. It will even be able to pick out these sound waves from a mixture of very dissimilar sounds. It is therefore sensitive to some and insensitive to others. It is the same with bodies struck by radiant heat. They only absorb certain vibrations and let others pass by them. I shall return to this point in the next chapter.
2. Permanence of the Radiation of Matter
Until the absolute zero is reached, matter unceasingly sends vibrations into the ether. A block of ice may therefore be considered as much a source of heat, and for the same reasons, as a fragment of glowing coal. The only difference between them is in the quantity radiated. The frozen plains of the Pole are a source of radiant heat like the burning plains of the Equator, and if the sensitiveness of the photographic plate were not so limited, it would be possible in the very darkest night to reproduce images of bodies by their own radiations, when refracted by the lenses of a camera obscura.
Naturally, these radiations, which all bodies constantly emit, only act on the thermometer when it is plunged into a medium colder than itself. If the instrument is first placed in a refrigerating mixture, capable of lowering the column of liquid to a level corresponding to -50° C, and then placed in front of a block of ice at 0° C, the heat radiated by this block will raise by 50° — that is to say, will bring back to zero — the temperature of the instrument. But if this last already marks zero — evidently no movement of the column of liquid can reveal the radiation. The ice would continue radiating on the thermometer and the later on to the ice, but they would only be exchanging their radiations. The radiation would therefore nonetheless go on, though marked by this exchange. When we say that a body becomes cool by radiation, we necessarily imply that it is plunged into a medium with a lower temperature than its own. Receiving from the latter less heat then it imparts to it, its temperature is lowered until that of the two bodies is equal.
When we are obliged to keep at a low temperature a body which is to be placed in a medium of higher temperature, we surround it with substances impermeable to radiation, and thence called athermanous. Wool and furs possess this property. Pictet has shown that for temperatures below -70 C the majority of athermanous bodies lose their properties and become diathermanous. We can only keep air liquid by enclosing it in double-walled vessels, between the walls of which a vacuum is made, and the inner surface of which is silvered [Dewar flask]. These vessels can also be used to keep liquids very hot, since they prevent the absorption as well as the emission of radiation.
3. The Electric Emissions Which Accompany Heat
We have just seen that matter is always absorbing and radiating. The exchange between it and the ether never ceases. The vibrations of the ether intercepted by matter are subjected by it to various transformation of a mechanism unknown to us, and of which we only perceive the extreme terms.
I have never ceased to insist in this and my preceding work on the relations of the ether with mater. They again appear when we examine the electric phenomena accompanying the caloric variations of bodies.
Physicists have had for a long time an inkling of the kinship between heat and electricity, and recognize more and more that the production of the one is accompanied by the simultaneous manifestation of the other. A body which is subjected to friction generates both heat and electricity. The heat which is propagated throughout a wire by the simple twisting of it on itself, generates electricity. A substance which liberates heat when combining with another, liberates electricity at the same time.
It is known also that the electric and caloric conductivities are sensibly in the same ratios for all metals. Those which are good conductors of heat are the same for electricity, and conversely. The chief difference lies in the speed of propagation. Immense in the case of electricity, this is, on the contrary, very slow in that of heat.
If heat easily transforms itself into electricity, the latter no less easily transforms itself into heat. It suffices to pass a current through a metal wire to see the latter become more or less red-hot according to its resistance. If a current be sent through a conducting wire half platinum and the other half silver, the platinum becomes white-hot, while the silver wire, a tenfold better conductor — that is to say, offering less resistance to the passage of the electricity — remains dark.
The recent researched mentioned in my last book make it possible to follow much further the course of this analysis. We now know that when, by one means or the other, a body is made incandescent, it emits not only radiant heat and light — which are, moreover, exactly the same thing –but, in addition, torrents of electric particles. We have even reached the point of admitting — an hypothesis which the experiments of Zeeman seem to confirm — that a flame consists only of electric particles in vibration. The movements of these electrons, propagated in the ether, would generate radiant heat and light. It is, however, very possible that the liberation of electric particles which accompany incandescence and many other chemical reactions, is only in many cases a secondary phenomenon, a king of unutilized excess of energies employed in modifying the equilibria of matter.
A constant relation ought to exist between the intra-molecular and intra-atomic energies. Atoms represent the stones of which the molecular edifices are built. In all the operations of ordinary chemistry, we simply displace these stones, and this is, no doubt, why the quantities of heat or electricity then brought into play are always met with again. When by various means, very inadequate as yet, we touch the structure of the stones of the edifice — that is to say, of the atoms — we liberate, in the form of heat, or electricity, quantities of intra-atomic forces of which the magnitude will vary according to the disturbances of equilibrium produced, and may bear no relation to the causes of such changes.
The whole of this and the preceding chapter are, if looked upon as explanations, evidently insufficient. Notwithstanding all the formulas with which it bristles, this region of physics is extremely obscure. The problem of heat is one of the most difficult, because its solution demands the knowledge of things which are as yet very difficult of access.
Chapter III – Transformation of Matter into Light
1. The Emission of Light by Matter
Light is produced by vibrations of matter propagated under the form of waves in the ether. When these waves possess a length suitable for impressing the eye, we give them the name of visible light. We call them invisible when the retina, which is only impressed by a small part of the whole extent of the solar spectrum, remains insensitive to their action.
Whether it is the vibrations producing the sensation of blue or red, or those which are without action on the eye, such as infrared or the ultraviolet, that are in question, they are all of the same species, only differ by their frequency, and all deserve the name of light. From this general definition there springs a first consequence. We ought to give the name of light to the visible or invisible radiations emitted by matter at all temperatures down to the absolute zero as we have seen when studying radiant heat.
Matter, then, is incessantly transformed into light at all temperatures. An eye with a retina sensitive enough would see in the dark all objects as if surrounded by a luminous halo, and darkness would be unknown to it. Such an eye perhaps does not exist, but different instruments allow us to make a substitute for it.
Let us examine some of the conditions of the transformation of matter into light.
When we heat a body, the vibrations of its particles become more rapid, and its emission of ethereal waves increases. These waves, at first too long to be perceived, as they get near 500° C are short enough to become visible and to give the sensation of red. From 800° to 1000° C still shorter waves appear, and the radiations emitted comprise the whole length of the spectrum. Their slight amplitude alone prevents us from perceiving them. Temperature acts especially by increasing the amplitude of the waves emitted, which renders them visible.
At each temperature, the heated body emits waves different in length according to its nature. The brilliancy of flames depending at equal temperatures on the ratio between the long and short waves emitted by the incandescent body, those sources of light which emit many more of the second than of the first will be the more luminous. The brilliancy of the Auer mantle is due to the weakness of its emissive power in the red and the infrared compared to its power of emission in the visible spectrum. The temperature (about 1650° to 1700° C) does not in this case differ notable from that of a simple gas burner.
As regards the brilliancy of the light, there would be no advantage in raising too much the temperature of a body, because we should then produce, as is the ease with the electric arc, invisible ultraviolet rays. The more, in fact, the temperature of a source of light is raised, the more the radiations it emits are displaced towards the ultraviolet.
The radiation of bodies generated by heating is produced by all actions capable of increasing their vibrations, especially those chemical reactions which furnished the early modes of lighting. As a type, we may quote the combustion of ordinary gas. Formed by a mixture of hydrogen and of carbides of hydrogen, it combines violently with the oxygen of the air when lighted. The particles of carbon from the carbide, being liberated and brought to incandescence, give the flame a brilliancy which pure hydrogen does not possess. Gas therefore owes its brilliancy to these incandescent particles held in suspension. Any solid body whatever — platinum, for example — might replace the particles of carbon.
In reality, the phenomena which takes place in any kind of flame — that of a simple candle, for example — are quite otherwise complicated. So much is this the case that we might consider a body in combustion, such as a lighted candle, as one of the phenomena in physics most difficult of explanation, and involving the solution, of which we have yet hardly a glimpse, of the problems relating to the dissociation of matter. All incandescence is accompanied, in fact, by the liberation of a torrent of electric particles comparable to the cathode rays or the emissions of radium. This liberation necessarily implies a commencement of that disaggregation of the atom which was formerly ignored, because the provision of energy contained in matter is so immense that the loss of it during combustion then passed unperceived.
This dissociation of the atom in a flame has been made apparent not only by the production of electric particles proceeding from this dissociation, but likewise by the deviation of the electrons of flames by a magnetic field; it has as its consequence the doubling of the spectral rays if the flame acted on by the magnetic field.
2. The Influence of Wavelength and Amplitude on the Action of Light
A body thrown into the water produces on its surface a series of concentric circular waves comparable to small parallel hills separated by valleys. The distance from the top of one hill to another is what is called the wavelength; the height of each hill from the bottom of the valley represents the amplitude of the wave. It is the same with light, the sole difference being that the undulations take place in the ether instead of being produced in a liquid.
The length of the wave and its height constitute two very different things which we must keep distinct if we wish to understand certain actions of light.
Whether it is a question of sound or of light, or of any periodical disturbance of any fluid whatever, the wavelength is an element of invariable magnitude during the whole period of a vibration, while its amplitude may vary within wide limits. Waves lose their amplitude by propagation, but their length, and consequently the number of vibrations per second, remain the same. The analogy with the oscillation of a pendulum is complete. Move a pendulum much or little from the vertical line, and the distance it travels in its oscillating trajectory may be very short or very long, but the time taken to effect it will be invariable, and will depend solely on the length of the pendulum.
What is the part played by these two elements, wavelength and amplitude? In the case of the pendulum, the vis viva [kinetic energy] of its waves increases with the amplitude of its vibrations. As regards sound, it is the wavelength that determines the pitch of a given note, while the amplitude determines the intensity of this note.
With light the undulation of the ether give, according to their length, notes which we call blue, red, green, etc. Their length is strictly invariable for each note; but their intensity may vary enormously with the amplitude of the waves emitted — from 1 to 1 million between 600° and 1800° C, for instance, in the case of red radiations according to the measurements of M. Lechatelier. The intensity of a radiation will make it oscillate between darkness and a blinding flash, without the wavelength undergoing any change.
Besides the temperature, there are different means of increasing or decreasing the amplitude of the ethereal waves, and consequently the intensity of a pencil of light. To do so, it is enough to concentrate or, on the contrary, to disperse it, by lenses of suitable form. The intensity of a note or of a color is then very variable, but the length of the waves which produce this note or color remains absolutely the same during the whole period of the vibrations.
The eye and the ear are not organized so as to accumulate impressions. A color or a note of a given intensity will always produce the same effect, whatever the duration of their action. It is otherwise for certain reagents — the photographic plate, for instance — capable of accumulating impressions. We can thus, with a very slight but prolonged intensity, produce effects identical with those obtained with a very great intensity, acting for a very short time. It is the possibility of this accumulation which enables us to photograph bodies having a phosphorescence invisible to the eye, simply because the amplitude of the vibrations of light emitted was too slight to impress the retina.
The sensitive plate sees the radiations emitted because it can accumulate them, and photographs stars which the eye does not see by reason of their too slight amplitude, although the sensitiveness of the retina is enormously superior to that of the plate. The eye is for light what the ear is for sound. There exist dark light and silent sound, which the eye and ear do not perceive, but which suitable reagents may reveal.
From the fact that those stars which are on the edge of visibility take an hour’s exposure to photograph, Deslandres remarks that “the relation between the sensitiveness of the eye and that of the photographic plate should be the ratio of 1/10 second and 1 hour, or (say) 1/40,000”.
Whatever be the reagent employed — retina, photographic plate, or chemical compound — there is always a minimum of amplitude variable in each, below which light has no action. Berthelot pointed out, for example, that the oxidation of bisulfide of carbon, which in the sunlight can be effected in a few hours, is never effected in diffused light, even in a year’s time. For other reactions, such as the combinations of chlorine and hydrogen, the intensity of light may, on the other hand, be extremely slight. These are phenomena which are not always taken into account, but which must be known in order to understand the effects of light. It is because they have been misunderstood that the variations of certain vegetable functions in the different regions of the spectrum have given rise, as we shall see, to so many contradictory interpretations.
3. The Invisible Spectrum
The researches effected during recent years have proved that the invisible solar spectrum is much more extended than the visible. While this last only reaches from 0.4 to 0.8 microns, the invisible spectrum goes a little beyond 5 microns according to Langley [who used a bolometer] — that is to say, it is about 12 times as long as the other. The invisible spectrum of artificial sources of light is more extended still, since, according to Rubens, it stretches as far as 60 microns.
The plates of the solar spectrum published in textbooks give a very false idea of it, Not only do they reproduce nothing but the visible region, but the distribution of the color in it is very inexact, inasmuch as the prismatic spectra used as models reduce to a fourth or a fifth of its real size the extent of the red, and much exaggerate that of the violet rays.

The distribution of the colors is only exact with the diffraction spectra obtained by means of gratings. The distance between the rays then being proportionate to the wavelength, the red occupies a much more considerable extent than in the spectra obtained with a prism.
It was in fact the employment of prisms for the production of spectra which led us to interpret inaccurately the position of the maximum of caloric energy. It was formerly placed in the infrared. We now know that it is found in the luminous part of the spectrum. But as, in comparison with the total length of this last, the visible region is of very small extent, it follows that the total caloric energy is much greater in the invisible infrared. According to the last measurements of Langley, the visible solar spectrum only contains one-fifth part of the caloric energy of the infrared region. The invisible region of the spectrum constitutes, then, the most important portion of light. It is only the sensitiveness of the human eye which creates the division between the visible and the invisible parts of the spectrum. It is not, doubtless, the same with all animals.
This immense invisible region of the spectrum, wherein is found the greatest part of its energy, ought to play a very important, though hardly suspected, part in the phenomena of vegetable life and in meteorology. We as yet know none of its properties except the caloric action. Its variations probably have considerable effect in the changes of the seasons. Langley has recognized that the solar spectrum changes at the different periods of the year, and that the distribution of its energy is not the same at different seasons.
The one-fifth of the solar radiation which appears under the form of visible light seems at first a very small proportion of the whole. It is in reality very great if we compare it with that of artificial light. According to Wedding’s latest researches (1905) all artificial sources of light, including the electric arc, utilize hardly 1 percent of the radiations produced.
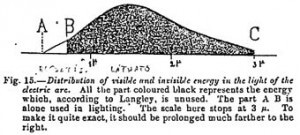
99% of the radiations emitted are, then, invisible.
Although these figures vary with the observers, none of them have found a less loss than 90%. If, then, we estimate at 4 million pounds, as has been done, the annual expenditure on artificial light of a great country like England, we shall see that the discovery of a means of transforming the invisible caloric energy into visible light would effect a saving of nearly 3 million pounds a year for one country alone.
The problem does not appear at all insoluble, since nature has already found the solution of it. The light of phosphorescent animals is almost exclusively composed of rays belonging to the visible region of the spectrum. All phosphorescent bodies also produce light without previous heating. It is probable that in this case, then, it is the energies of the atoms and not the disturbances of the molecules — as in the case of incandescence — which come into play. We will return to this point when studying, in a future chapter, the phosphorescence of gases.
4. The Distribution of Energy Throughout the Spectrum
The distribution of energy in the various regions of the spectrum has been the object of numerous researches. They have not led, however, to any very useful results, for the simple reason that, at a uniform temperature, the intensity of the various radiations varies greatly according to the source of light. We have seen, for example, that the distribution in the spectrum of a gas burner and in that of an Auer mantle was very different, although their temperature was almost identical.
Nor is there any profit to be drawn from the published researches on the distribution of energy in the solar spectrum, by reason of the very great variation which takes place in the infrared according to the days, the hours, the attitude, and the absorption exercised by the greater or less quantity of water vapor in the atmosphere, etc.
All the early measurements of the energy of the spectrum were, moreover, affected by errors due to the employment of the prism to separate the various radiations. As the prism heaps together the radiations in the infrared, and spreads them out considerably at the other end of the spectrum, it was natural that the heat should be very great in the part where the radiations were most condensed. We therefore supposed that in the infrared was to be found the hottest part of the solar spectrum. This error and the curve which represents it still figure in most elementary treatises on physics.
As soon as we succeeded, by means of gratings, in producing spectra in which the deviation of the radiations is proportional to the wavelength, it became evident that it was not in the invisible infrared, but in the most luminous part of the spectrum, that is from A to D, that the maximum of caloric action in the light of the sun is to be found.
No part of the spectrum is really devoid of caloric action, as physicists for a long time believed. It would suffice to give to any radiation sufficient intensity to cause it to produce any caloric action one could desire.
Independently of the causes of error just enumerated, there is one much more serious still, which is connected with the very principle of the materials used.
Physicists were led to measure the energy of the spectrum solely by the evaluation of the caloric notion of its different parts. M. Jamin (Physique, 4th edition, t. iii, p. 160) shows in the following passage the mental process involved in that conception: —
“It was formerly supposed that three distinct agents emanated from the sun — heat, light and the chemical rays — and that each of these gave rise to a spectrum partially superposed upon the two others, but as distinct in its nature as in its properties. But we have been led by the force of events to reject this complicated hypothesis, because all experiments proved powerless to realize in practice the separation supposed to be possible in theory. Everybody now admits that the sun sends us vibrations, which are all of the same nature, and which are only distinguished from each other by their wavelengths. These different actions (luminous, chemical, and caloric) are all executed at the expense of the energy of the vibrations, but the caloric action supplies the only rational measurement of them”.
This mode of measurement was applied not only to light, but to all forms of energy. It is deduced from the idea that all the modes of energy, being capable of transformation into heat, are measurable by their caloric effects evaluated in calories or kilogram-meters, which are consequently their equivalents.
By considering in the spectrum nothing but heat, we were naturally led to attribute to it all the actions observed. This is exactly what the last-quoted author did. “It is at the expense of the calorific energy of which the radiations can dispose”, he says, “that the impression of light on the eye and that on the photographic plate are alike produced”. If it were really so, the rays which can produce the maximum of heat ought to be those which can best act on the photographic plate and on the eye. Now, it is just the contrary which is the case. It is not only in the photographic action that we notice this want of parallelism between the caloric intensity and the effects observed. It is really striking to see produced in very energetic fashion, in the ultraviolet, the caloric action of which is almost nil, certain effects, such as the dissociation of matter, while they are insignificant in the hotter parts of the spectrum.
We ought to conclude from this, that the various regions of the spectrum possess actions having no common measure. According to the reagent employed — the eye, the photographic plate, the thermometer, the electrometer — the distribution of energy will be very different. The reagents strongly impressed by a certain radiation are silent to another.
In reality, a curve is required for each of them, and it must not be claimed that the energy of the spectrum can be determined by a single one, as has hitherto been done.
5. The Absorption of Light by Matter
To its property of emitting luminous rays, matter adds that of being able to absorb them or to allow them to pass through it. There is thus a permanent interchange between matter and the ether, as I have already pointed out.
When a body allows light to pass through itself it is called transparent. In the contrary case, it is called opaque.
Our ideas as to transparency and opacity have been much modified during the last few years. It is known at the present day that there is no body entirely transparent to all radiations. A strip of glass one-tenth of a millimeter thick, completely transparent to the eye, is yet absolutely opaque for the whole ultraviolet extremity of the spectrum and for a notable part of the infrared.
Transparency is always selective and, consequently, never complete. If there existed a body entirely transparent, it could be exposed to the most intense source of heat without becoming warm, since it would not absorb any radiation. The rise in temperature of a body exposed to a radiation is only due, in fact, to the absorption of it of those radiations which have no temperature of their own.
The greater the opaqueness of a body the more it absorbs and the more it becomes heated, except, naturally, in cases where, through the polish of its surface, it sends back into space the vibrations of the ether which reach it.
We now attempt, as said before, to explain the transparency and opacity of bodies by a phenomenon resembling that of acoustic resonance. A slight modification of the current theory will suffice to show that these phenomena are the consequences of the same law. Matter may be considered to be composed of small molecular tuning forks capable, like ordinary ones, of vibrating to certain notes, but not to others. When struck by the vibrations of the ether, they vibrate according to their structure, in unison with certain vibrations and receive no impressions from the others. The radiation which causes them to vibrate remains, in issuing from the transparent body, exactly the same as when it entered, having undergone, in the case of other modifications, only a slackening of speed, in consequence, no doubt, of the time necessary to increase the vibrations of the atoms.
Opaque bodies must, on the contrary, be formed of elements unable to vibrate in unison with the vibrations which strike them. They can, then, only emit irregular vibrations which vanish at once by transmitting themselves to the neighboring molecules. From these movements must result the heating of bodies struck by light. Absorption would be, then an absolute transfer of the movement of the ether to the bodies which are plunged therein.
When a substance is transparent to one radiation and opaque to another — which is the general case — the molecules vibrate in unison with the vibrations which pass through them and absorb others. A red or blue glass possesses its color because it can only allow the radiations of the spectrum corresponding to the blue or red to pass and can keep back the others.
This theory of resonance is only maintainable if we suppose the molecules of bodies to be already animated by rapid movements to which the vibrations of the ether do but impart direction. It would be, otherwise, quite impossible to suppose that the vibrations of the ether could give to the atoms the enormous total of energy necessary to cause them to oscillate with the rapidity of light.
According to this theory, the only difference between a transparent and an opaque body rests on the nature of the vibrations they each emit. The vibrations falling upon them pass through the transparent body, and cause in their passage the atoms of matter to vibrate with the incident ray. They would equally cause the atoms of the opaque body to vibrate, but would diffuse themselves throughout its mass. In both cases the luminous energy, having struck the face of an opaque or a transparent plate, necessarily reappears on the other side. In the case of transparency, the ray on issuing is the same as on entering; in the case of opacity, the plate is heated, and then emits in all directions — and no longer in one single direction — radiations with a wavelength greatly differing from that which struck the other side. While the light does not modify the temperature of a transparent body, it raises, on the contrary, that of an opaque one.
If the amplitude of the luminous waves striking an opaque body be great enough, the molecules of this body may be driven sufficiently apart to cause it to pass into the liquid or gaseous state.
The absorption of light by matter is closely connected with the structure of this last. The modifications produces by its changes enable the composition of bodies to be ascertained. They are easily noticed by interposing the substance it is desired to examine between a luminous source and the prism of a spectroscope. Many liquids very transparent to the eye present bands of absorption which vary with the slightest changes in their composition. Traces of impurity are thus easily discerned. It is possible, for instance, to detect the presence of 1/50,000 part of pyridine in ammonia.
Gases are very absorbent for certain radiations, and very little so for others. He gases of the atmosphere totally absorb all the ultraviolet starting from 0.295 microns, and all the infrared beyond 5 microns. The ozone of the atmosphere also shows itself very absorbent for the ultraviolet, and the accidental disappearance of this region from the line M onwards, observed in my experiments, is perhaps due to the momentary excess of this substance.
It is somewhat difficult in the theory of transparency by resonance, given above, to understand how the same body can be transparent or opaque to regions situated at the extremities of the spectrum. Window glass is quite opaque, not only to the ultraviolet, but likewise to all the infrared region beyond 2 to 3 microns, and consequently to the caloric radiations emitted by bodies heated to 100 C or less.
This partial transparency of matter to the luminous vibrations of the ether should be compared with its complete transparency to the electric or magnetic lines of force pointed out previously, which are also composed of ether, but in a form unknown to us. They are the only elements of which matter is unable to stay the progress. Why does it allow the ether to pass in one form and not in another? I can give no answer to this question.
6. The Chemical and Photographic Action of Light
When the vibrations of the ether produced by the heating of matter meet a body, they produce varied effects, which we may divide into three classes.
- Mechanical Action ~ This is the pressure exercised by radiant energy. Being very slight, it can only be made evident by very sensitive instruments. It may, however, be sufficiently intense to annul gravity in the case of very light bodies. The deformation of comets is attributed to its influence.
- Dissociating Action on the Atoms of Matter ~ This is put in evidence by the researches set forth in my former work, to which I shall return in a later chapter.
- Chemical Actions ~ Comprise very different reactions (oxidation, reduction, etc) made use of in photography.
Of these different actions, I shall now only study certain particular effects with regard to photography which have been observed in the course of my researches.
It being conceded that the electric particles of the cathode rays and of radioactive bodies impress photographic plates, we might be tempted to ascribe the formation of the latent image to a kind of ionization of the gelatino-bromide of silver. I formerly thought of maintaining this hypothesis, but there are the two following facts against it: (1) It is impossible to observe any radioactivity during the exposure of the photographic plate to the light; (2) the blue rays which chiefly act on the photographic plate are by no means the most active agents in the dissociation of matter.
The elucidation of this last point led me to inquire which were the radiations with the greatest action on the photographic impression.
In order to make these evident I exposed a photographic plate behind a spectroscope, and watched in which region the impression commenced. It always began in the blue, and never in the violet or the ultraviolet.
Although the Jena-glass prisms of my spectroscopes allow nearly all the solar ultraviolet to pass, they absorb, however, a portion, and it might be objected to the above experiments that the feeble action of the violet and the ultraviolet was the result of this absorption. I therefore asked M. de Watteville, who owns a large spectroscope with a quartz prism, very transparent to ultraviolet rays, to repeat my experiments with his instrument. They gave results identical to those set forth above. The impression always begins in the blue, and is only propagated some time afterwards into the ultraviolet. It may be gathered from this that the use of objectives of quartz or of glass very transparent to the ultraviolet would offer absolutely no advantage in instantaneous photography.
It would be much more interesting to increase the sensitiveness of the plates for all regions of the spectrum. The part utilized in photography hardly represents more than about the 20th part of the solar spectrum, which goes from 5 microns to 0.295 microns, including the visible and invisible rays. The visible part only extends from 0.4 to 0.8 microns. Even if we only take note of the visible spectrum, it will be seen that the photographic plate utilizes but a very small part of it.
No doubt, by various means, we can render the plates fairly sensitive as far as the red; but this sensitiveness is very illusory, for enormous differences of exposure are always required to obtain images with blue, green, and red light. The only real advantage of the pates termed monochromatic is that they are less sensitive to the blue than ordinary plates. The same result is obtained by simply placing a yellow glass before the objective. With any plate whatever we obtain an impression as intense as can be desired by sufficiently prolonging the exposure. A landscape may very well be photographed through a red glass.
It is this difference of rapidity of action in the different luminous radiations which changes all the values in the photographic reproduction of landscapes. We obviate these differences somewhat by prolonging the exposure so as to allow the feebly actinic rays time to act, but then soon comes in the phenomenon of irradiation consisting in the fact that every part impressed acts as a luminous center on the neighboring region, not only directly, but by its reflection on the posterior of the glass. I have made not a few experiments on this subject, and have observed that with sufficiently long exposures the impression can be propagated to within half a centimeter of the region reached by the light. It is for this reason that the photography of very fine lines is very difficult. Vain attempts have been made to thus reproduce by photography the diamond-cut gratings employed in certain processes.
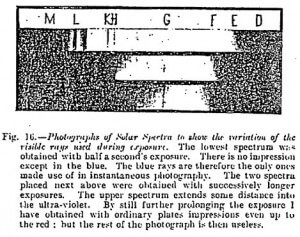
I will sum up for my photographer readers the experiments that I have carried out — with the spectroscope described in another chapter — for the examination of those regions of the spectrum which impress photographic plates according to the length of exposure, the nature of the plates employed, and the colored glasses placed before the objective.
Parts of the Solar Spectrum utilized in photography according to the length of exposure with ordinary and with orthochromatic plates (Cf. Figure 16)
(1) Ordinary rapid plates: Instantaneous exposure — The impression extends from F to the middle of the interval between H and G; 2-seconds exposure — The image extends up to K on one side, and nearly to E on the other; 15-seconds exposure –The impression is prolonged beyond L on the side of the ultraviolet, and extends nearly up to D on the side of the red.
It is, then, sufficient to expose an ordinary plate long enough for it to be impressed by the least actinic rays. In proportion as the exposure is lengthened, the intensity of the impression increases between H and E, and much less rapidly from E to A. We then have a plate over-exposed for the blue, and hardly enough so for the other colors.
Influence of a colored glass — A blue glass reduces but very feebly the image during instantaneous exposures even with the ultraviolet region. A yellow glass does not reduce the intensity of the image on the side of the red, but does so on the side of the blue, i.e., from H to the ultraviolet. If instead of 1 second’s exposure we give it 30 seconds, all the colors impress the plate, and, moreover, as the action of the blue is much extended, the unevenness of the impression is diminished. In photography therefore, as soon as one has to prolong the exposure, a dark yellow glass should be put in front of the objective. The best orthochromatic plate is an ordinary one with a yellow glass. A green glass would further reduce the impression on the side of the blue, but its use would only be of advantage with a too prolonged exposure.
(2) So-called orthochromatic plates: The substances with which these plates are covered render them much less sensitive to the blue rays than ordinary plates. Their sensitiveness extends from a little on the side of the red to beyond D, but without in any way attaining the ray A save with an exaggerated exposure. These plates behave in reality like an ordinary plate before which one has placed a yellow glass, but are very inferior to it.
They are very little sensitive in the green (between E and F), i.e., exactly in that region where sensitiveness is most necessary. Moreover, if their sensitiveness is greater than that of ordinary plates on the side of the red, it is much less so on the side of the violet. If one uses them with a yellow glass, as has been proposed, the effects are disastrous. The impression between E and F, i.e., in the region of the green, from being insufficient is arrested altogether. Orthochromatic plates — at least those made in France, which I have alone studied — possess beside the above defects that of being foggy. Even when one develops them in complete darkness they give gray and flat images.
Chapter IV – The Dematerialization of Matter Under the Action of Light
1. The Dissociation of Matter Under the Influence of Different Radiations of the Solar Spectrum
I have studied at length, in The Evolution of Matter, the dissociation which all bodies undergo under the influence of luminous radiations, and have shown that a body struck by light emits effluves of the family of the cathode rays, of which the quantity varies considerably with the nature of the radiations. If I return to this question, it is because I have been led to study in this work the principal actions of light. My experiments on this subject were recently verified by one of the most illustrious scholars of the day, Sir William Ramsay (1).
[(1) “The work”, writes Sir William Ramsay, “was undertaken with the object of repeating some experiments of LeBon, described in several papers in the Comptes Rendus, and afterwards in greater detail in his treatise, The Evolution of Matter. It will be remembered that LeBon, by allowing ultraviolet to fall upon clean metallic surfaces raised to a high potential, caused them to give up their charges”. — Philosophical Magazine, October 1906, p. 401)
He has published, with regard to the dissociation of matter under the influence of light, a memoir extremely remarkable, not only on account of the precision of the experiments, but also of the theoretical considerations which it contains. The results obtained by him were identical with my own, and he entirely admits the theory of the dissociation of matter. His conclusions are even bolder than mine.
“If it turns out to be true”, he says, “as Soddy claims to have shown” that a disintegrating element which parts with beta rays or electrons, leaves behind it matter not associated with a positive charge; and if it be also true that such ‘disintegration’ implies transmutation into some other form of ‘elementary matter’, then it may be that the phenomena of which a description is given on the following pages refer to cases of transmutation. When zinc, for example, illuminated by uv light parts with corpuscles, it may be that the residual matter — the zinc minus electrons — is no longer zinc, but some other form or forms of elementary matter”. Ramsay considers the action of uv light, moreover, as a kind of detonator which produces the disintegration of the elements of matter.
I was very pleased to see so eminent a scholar confirm the correctness of my experiments, and arrive at the conclusions which I have so long upheld. It will not be without interest to state briefly the origin of these last.
The experiments by which I demonstrated that the action of light on bodies produced effluves similar to those of uranium — the only radioactive body then known — are not new, since they were published for the first time about 10 years ago. They were the starting pint of my theory of the universal dissociation of matter, and I have recurred to them in several memoirs.
After having shown that solar light exercised in different degrees a dissociating action on all bodies, I commenced the examination of uv radiations, the study of which had given rise to many works. My remarks demonstrated: —
(1) That the so-called negative discharge was likewise positive, contrary to what was then taught.
(2) That the discharge of electrified bodies is very different according to the bodies employed, a point likewise verified by Ramsay, and contrary to what was then taught.
(3) That it was not at all by the pulverization of the metal struck by light that the discharge was effected, as was formerly the opinion of Lenard, but by the dissociation of its atoms. This most important point, but little disputed at the present time, and likewise admitted by Ramsay, was very new and unforeseen at a date when no one dreamed of establishing a kinship of any kind between the effluves produced by the action of light and the cathode and uranium rays.
All these experiments, which appear so simple when one reads them set forth in a book, bristle with enormous difficulties, and, above all, with causes of error, which explain the erroneous opinions formulated by observers. They studied, moreover, the action of light on bodies without having ever suspected that from this study would one day issue the theory of the dissociation of matter.
Other persons will carry on these researches, for the subject is far from being exhausted. I shall render them service by pointing out the causes of error which delayed me for a long time, and finally led me to establish the spontaneous radioactivity of all the metals. Nothing would be more instructive for the history of the evolution of ideas than the recital of the uncertainties through which those engaged in research have passed, and of which their final works naturally contain no traces.
In my first experiment had indeed verified that the effluves emitted by bodies subjected to the action of light passed, as has been likewise recognized by Ramsay, through thin metallic screens. But as they sometimes seemed to transpierce somewhat thick ones, I had to seek the reason of this anomaly. The cathode rays, to which I assimilated these effluves, can, in fact, only pass through extremely thin plates.
I first observed that these effluves went round obstacles in the most curious way, as if they rolled on their surface. The remedy seemed very simple, since it was only a question of giving to the supposed screens the form of a closed cylinder surrounding the ball of the electroscope. But instead of simplifying the question I had created new problems, to interpret which took me several months.
The electroscope, surrounded by its protecting cylinder, on exposure to the sun discharged itself to the extent of several degrees in a few minutes, and then it gave no further discharge, even when the metal was cleaned. If the cylinder was replaced by another of the same substance the discharge recommenced, and then after a certain lapse of time stopped again. For what reasons did a body having certain properties lose them a few minutes later?
I will not enumerate here all the researches made to separate the factors which might be at work and to study the action of each. From one elimination after another, there remained only the influence of heat. This was indeed the active cause, for by replacing the sun by a body heated but not incandescent, and placed in the dark near the cylinder surrounding the electroscope, the discharge took place; but, as with the sun, it soon stopped. What part did heat play in this phenomenon?
Evidently it was possible that an amount of heat only capable of raising by a few degrees the surface of a metal should render the air contained in its interior a conductor of electricity. Heat, moreover, could not be the only element which intervened, since the metal cylinders exposed to its action soon lost their influence on the electroscope. No amount of cleaning restored their properties. The majority of them, however, regained them spontaneously in the course of a few days.
Again I had to proceed by successive elimination, and I at last succeeded in verifying that the metals lost under the influence of heat something which they cold afterwards regain by repose. This something was simply a small provision of radioactive particles formed spontaneously in all bodies. As the final result of these researches I reached the two following conclusions: — (1) Light, especially the uv rays, which only exercise, as is known, an insignificant caloric action, dissociates matter and transforms it into products analogous to those emitted by radium or uranium; (2) outside the action of light, and independently of it, luminous or dark heat provokes in bodies the loss of an infinitesimal quantity of the radioactivity they contain, which may be spontaneously regenerated. All bodies are therefore slightly radioactive, and the dissociation of matter is indeed a universal phenomenon.
Ramsay has very thoroughly observed in his skillful experiments this ‘fatigue’ of metals, which lose their properties more or less after a certain time. He attributes it to a modification of the equilibrium of the atoms on their surface, a theory which, however, does not sensibly differ from mine.
2. Origin of the Phenomena Attributes to the Presence of Radium
Since I am on the subject of radioactivity, it will not be without interest to say a few words on a great discussion which has been eagerly followed by the English public, and in which the most eminent scholars — Lord Kelvin, Sir Oliver Lodge, Sir William Crookes, etc. — have taken part. From the scientific journals it has passed into political papers like the Times. Though the engagement was sharp, the conclusions have remained very uncertain.
Its starting point was the extension of this theory, still very general but very erroneous, as may be seen by the above statements, that all radioactivity is due to the presence of radium or some body of that family.
Radioactivity being now found everywhere, physicists who do not yet admit the theory of the universal dissociation of matter are indeed compelled to suppose that there is radium everywhere. After having been the most rare body in nature, it should now be the most abundant.
Starting with this idea, a physicist maintained before the British Association that the internal heat of the globe might well be due to the action of the radium of which the earth should be full.
Persons who have not made a deep study of this body may think that it is a well-defined substance like sodium or gold, and that consequently it is easy to ascertain its presence by certain reagents; now, it is nothing of the kind. Let us put aside certain rays in the spectrum of a rather disputable interpretation, and which, moreover, are only observed in very concentrated solutions of salts of radium; and let us examine on what is based the assertion that this body is very common.
It is simply this fundamental characteristic — the emission of particles which bear a certain quantity of electricity, and are therefore capable of discharging an electrometer. There exists no other means of practical investigation, and it was by taking this exclusively as a guide that radium was finally isolated from the various substances with which it was mixed up.
This characteristic would, moreover, be an excellent touchstone if radium or the substance of the same family were alone present in it. But all bodies in nature possess it, as I have shown, either spontaneously or under the influence of very varied causes, such as light, heat, chemical reactions, etc.; and it follows that properties are attributed to radium which may belong to very different bodies. If we are bent on admitting that radioactivity is the cause of the internal temperature of the globe, there is no need to invoke to supposed presence of radium. All bodies at a high temperature, such as are apparently those which exist in the interior of our planet, liberate torrents of electric particles analogous to those produced by radium. I do not know whether they serve to maintain the earth’s heat, but it seems more reasonable to believe that they play a part in the production of earthquakes.
That which concerns the actions of light on matter may be summed up in the statement that the light absorbed by a body transforms itself, according to that body and to the rays which act on it, into very different effects — light, heat, chemical equilibria, dissociation of matter, etc. In the case of dissociation, the energy emitted by the dissociated body in the form of different particles may be far superior to the energy which provoked its dissociation. Light, then, acts like a spark on a mass of gunpowder. It may therefore be said in a general way that all the physical or chemical properties of a body which has absorbed light are more or less modified by the fact of this absorption alone.









